Chemistry Nobel Prize 2022: Barry Sharpless, Morten Meldal and Carolyn Bertozzi, who won the 2022 Nobel Prize in Chemistry on Wednesday, made important breakthroughs that made it possible to create new molecules efficiently. Creation of new molecules is vital for pharmaceuticals, as well as materials science.
The Royal Swedish Academy of Sciences awarded the Chemistry Nobel to the chemists “for the development of click chemistry and bioorthogonal chemistry.”
American chemist Sharpless has become the fifth individual to be awarded two Nobel Prizes. He was also awarded the 2001 Nobel Prize in Chemistry.
Sharpless, who has helped the W. M. Keck professorship in chemistry at The Scripps Research Institute, California, since 1990, pioneered a new kind of chemistry which he called click chemistry.
Danish chemist Meldal, a professor of Chemistry at University of Copenhagen, Denmark, discovered a reaction that showed how adding certain compounds can result in a click chemical reaction.
Bertozzi used click chemistry to develop reactions that can occur inside living organisms, and created a new type of biological pharmaceutical to attack tumour cells. Using bioorthogonal reactions, Bertozzi took click chemistry to a new dimension.
Why should new molecules be created?
Creation of new molecules is necessary to develop therapeutic drugs and pharmaceuticals. When complex molecules are built, several by-products are created. These reactions take place in multiple steps, and the by-products can interfere with the efficiency of the process. Therefore, it is important to remove by-products to ensure a smooth flow.
However, removal of by-products is challenging because it is a time-taking and expensive procedure, and leads to the loss of other materials. This is where click chemistry comes to the rescue.
What is click chemistry?
Click chemistry is a term that was coined by Sharpless in 2001 to describe reactions that produce a high yield, wide in scope, create only by-products that can be removed without separation techniques such as chromatography, are simple to perform, and can be conducted in easily removable solvents.
Click chemistry is a technique that allows molecular building blocks to snap together quickly and efficiently, the Nobel Prize Organisation says on its website. It is used to develop pharmaceuticals, map DNA and create new materials.
What are bioorthogonal reactions?
A bioorthogonal reaction is a reaction taking place within a living system which does not interact or interfere with the native biochemistry of the system. These reactions, which occur without disturbing the normal chemistry of the cell, have huge implications for biochemistry.
Some of the applications of Bertozzi’s bioorthogonal reactions include targeted cancer treatments, among others.
Sharpless found a simple way to create molecules
Before receiving his first Nobel Prize in Chemistry in 2001, Sharpless was working on a new approach in chemistry which. He wrote in a scientific journal that he believed it was time for chemists to stop imitating natural molecules because the processes were complex, creating obstacles to the development of new pharmaceuticals.
Citing the example of a powerful antibiotic, meropenem, Sharpless explained that a level of production efficiency higher than that used in clinical trials is necessary for the industrial production of a particular substance. After six years of work, Sharpless found a way to produce the antibiotic on a large scale.
According to Sharpless, the bonds between carbon atoms are a stumbling block for chemists. This is because carbon atoms from different molecules lack a chemical drive to form bonds with each other, as a result of which they need to be artificially activated. The artificial activation often results in unwanted side reactions and loss of material.
Therefore, Sharpless encouraged his colleagues to try and link smaller molecules that already had a complete carbon frame, instead of forcing reluctant carbon atoms to react with each other.
ALSO READ | Nobel Prize 2022: Relationship Between Humans And Extinct Relatives – Discoveries That Won Swedish Geneticist Physiology Nobel
It was observed that the simple molecules could be linked together using bridges of nitrogen or oxygen atoms, which are easier to control. In these reactions, there is a strong intrinsic drive for the molecules to bond together. As a result, side reactions can be avoided, and the loss of material will be minimal.
According to the Nobel Prize Organisation, Sharpless said that even if click chemistry cannot provide exact copies of natural molecules, it will be possible to find molecules that perform the same functions. Therefore, click chemistry can be used to produce pharmaceuticals on an industrial level, he said.
Sharpless wrote in a publication in 2001 that one of the criteria that should be fulfilled for a chemical reaction to be termed a click chemistry reaction is that the process should occur in the presence of oxygen and water, a cheap and environmentally friendly solvent.
Meldal’s click reaction that revolutionised chemistry
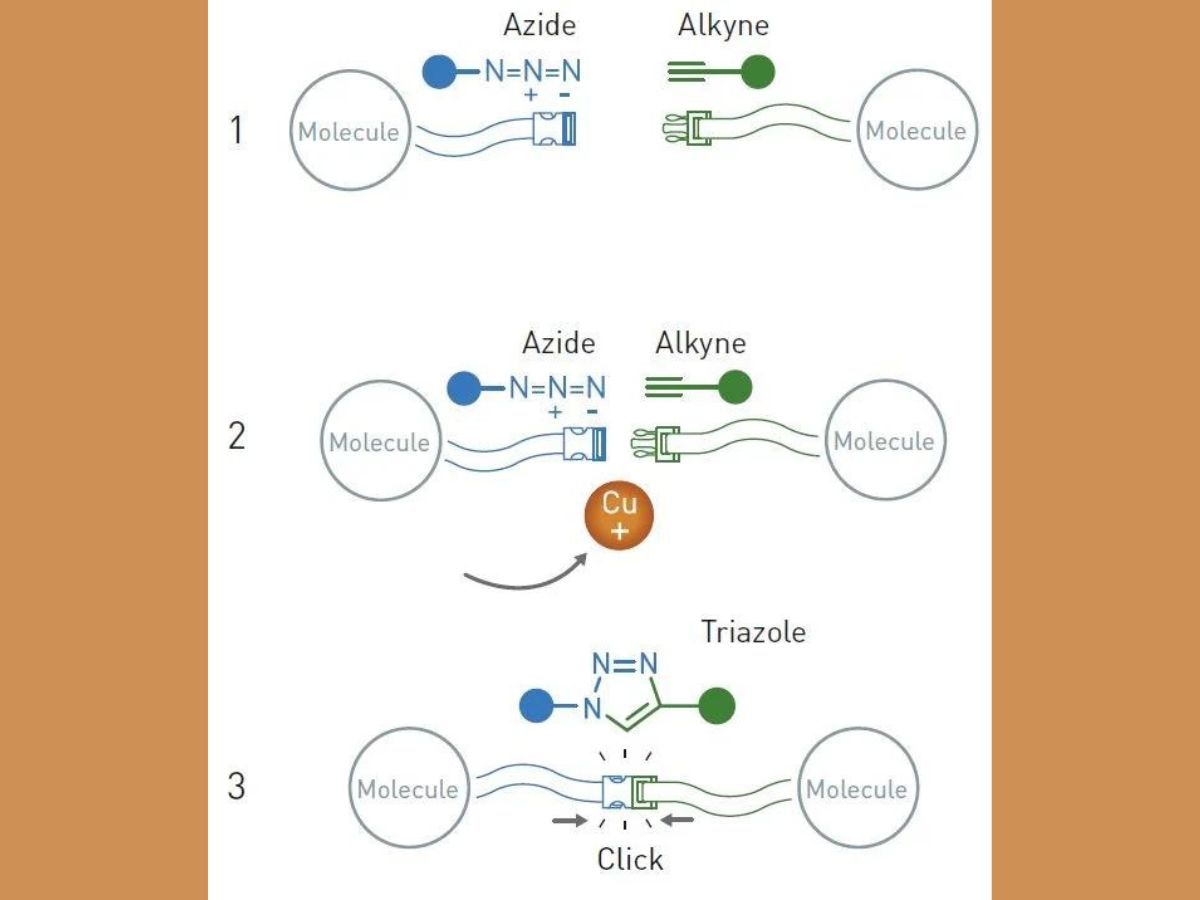
Meldal and Sharpless independently discovered a reaction that revolutionised chemistry, and is described as the “crown-jewel” of click chemistry. The reaction is called the copper catalysed azide-alkyne cycloaddition. An azide is a compound containing the N3 molecule, and alkyne is an unsaturated hydrocarbon containing a triple bond.
Meldel, while working on methods to find potential pharmaceutical substances, conducted a purely routine reaction, in which their aim was to make an alkyne react with an acyl halide, an organic compound containing a halogen atom. Scientists need to add copper ions and palladium as catalysts to ensure a smooth reaction.
However, Meldal and his colleagues observed an unexpected result. The alkyne reacted with the wrong end of the acyl halide molecule. That end had an azide. Together, the alkyne and azide molecules created a ring-shaped structure known as triazole.
Triazoles are useful chemical structures, stable, and found in some agricultural chemicals, pharmaceuticals and dyes.
Though scientists had tried to create triazoles in the past, the reactions also led to the formation of unwanted by-products. Since Meldal had used copper ions, the reaction was controlled, so that only one substance was formed. Therefore, the acyl halide remained untouched in the reaction vessel.
In June 2001, Meldel presented his discovery at a symposium in San Diego, and in 2002, he published his results in a scientific journal.
The same year, Sharpless, independently of Meldal, published a paper about the copper catalysed reaction between azides and alkynes, and described it as an “ideal” click reaction. The reaction works in water, and is reliable, Sharpless wrote.
Bertozzi’s work on glycans
In the 1990s, Bertozzi started working on glycans, a group of molecules that hardly received any attention in the past. Glycans are complex carbohydrates that are built from various types of sugar, often sit on the surface of proteins and cells, and play an important role in many biological processes, including viral infections and activation of the immune system.
An obstacle to chemists was that they did not have the tools to study glycans. However, Bertozzi decided to overcome the challenge, and in the 1990s, started mapping a glycan that attracts immune cells to lymph nodes.
ALSO READ | Physics Nobel 2022: The Mysteries Of Quantum Entanglement, And Their Relevance For The Future
Bertozzi heard from a German scientist at a seminar how he had succeeded in making cells produce an unnatural variant of silicon acid, one of the sugars that build up glycans. She decided to use a similar method to make cells produce a sialic acid with a type of molecular handle, so that she could use the handle to map the cells which incorporate the modified sialic acid.
She thought that she could attach a fluorescent molecule to the handle so that the emitted light would reveal where the glycans were hidden in the cell.
Therefore, she used bioorthogonal chemistry to illuminate the cell.
Bertozzi performed a copper-free click reaction in living cells
The copper catalysed azide-alkyne cycloaddition reaction allowed an azide to click onto an alkyne only in the presence of copper ions. Since copper is toxic to living beings, the reaction cannot be performed inside living creatures.
Bertozzi found that in 1961, there had been research which showed that azides and alkynes can react in an almost explosive manner, without the help of copper. This is possible if the alkyne is forced into a ring-shaped chemical structure, resulting in a strain that generates enough energy to ensure a smooth reaction.
Bertozzi tested the reaction in cells, and saw that it worked well. She published the copper-free click reaction, called strain-promoted alkyne-azide cycloaddition, in 2004.
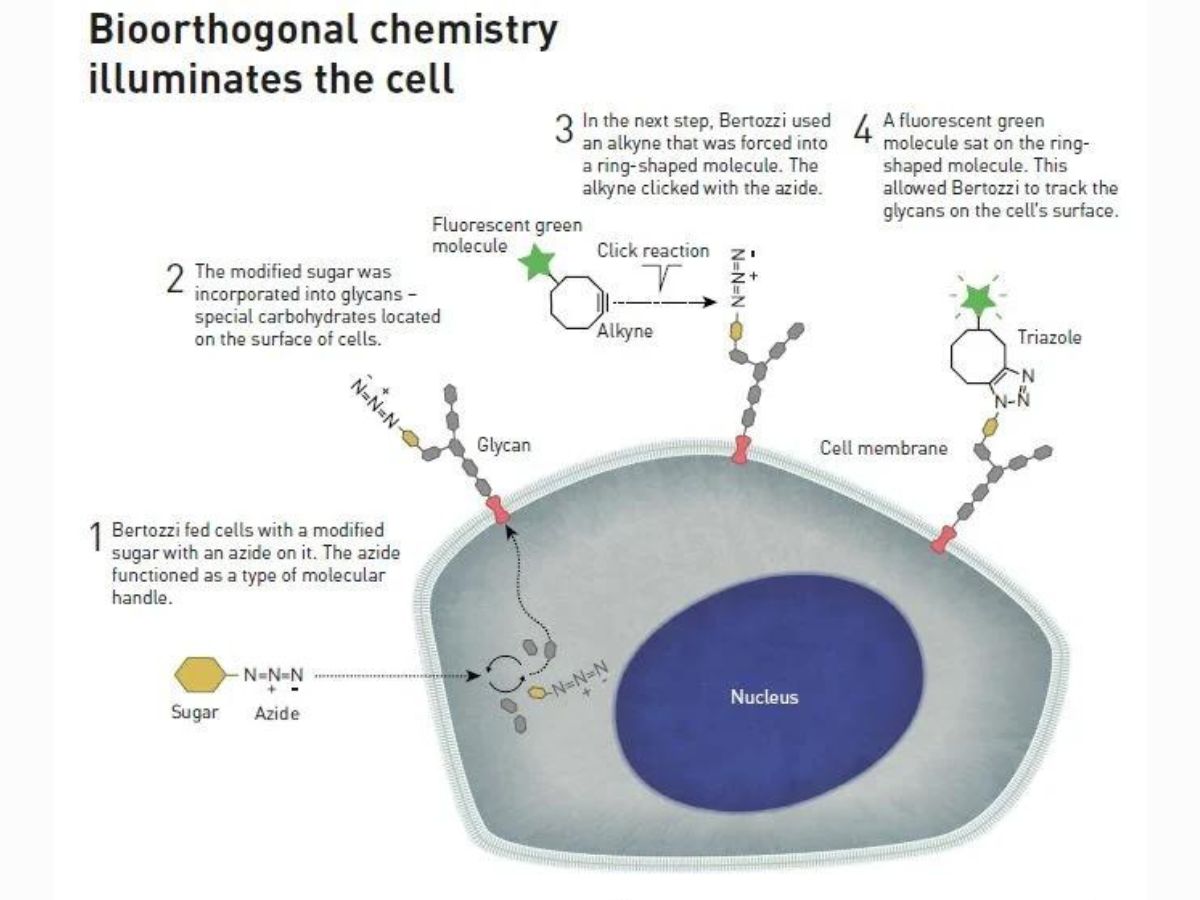
First, Bertozzi fed cells with a modified sugar with an azide on it, which functioned as a type of molecular handle. Then, the modified sugar was incorporated into glycans.
After this, Bertozzi used an alkyne that was forced into a ring-shaped molecule. A click reaction occurred between the alkyne and the azide.
She also attached a fluorescent molecule to the alkyne, allowing her to track the glycans on the surface of the cell.
The biggest advantage of this process is that azide does not affect living cells, which means it can be introduced into living organisms.
Why click chemistry and bioorthogonal reactions matter in pharmaceuticals
Click reactions are beneficial to the world because they can be used to produce new materials. For instance, some manufacturers add a clickable azide to a fibre or plastic, and click in substances that conduct electricity, are antibacterial, capture sunlight, and protect from ultraviolet radiation.
Click chemistry is also used in the pharmaceutical industry.
Bertozzi has studied glycans on the surface of tumour cells. She found that glycans appear to protect tumours from the body’s immune system, by making the immune cells shut down. Together with her colleagues, Bertozzi created a new type of biological pharmaceutical to block the protective mechanism.
In order to make the pharmaceutical, they joined a glycan-specific antibody to enzymes that break down the glycans on the surface of tumour cells.
Scientists are testing the pharmaceutical in clinical trials on people with advanced cancer.
Some researchers are developing clickable antibodies that target a range of tumours by attaching to them. After this, a second molecule that clicks to the antibody is injected. This molecule could be a radioisotope that can track tumours using positron emission tomography (PET) scanners. A molecule aiming a lethal dose of radiation at the cancer cells could also be used.
In this way, click reactions and bioorthogonal chemistry are of huge benefit to humankind.







